Top > About Us > News Letter > Supporting the foundation of clinical research
Supporting the foundation of clinical research
Current medical practice at the University of Tokyo Hospital
Central Office for Development of Advanced Medicine, URA, Takaaki Goto, Ph.D.
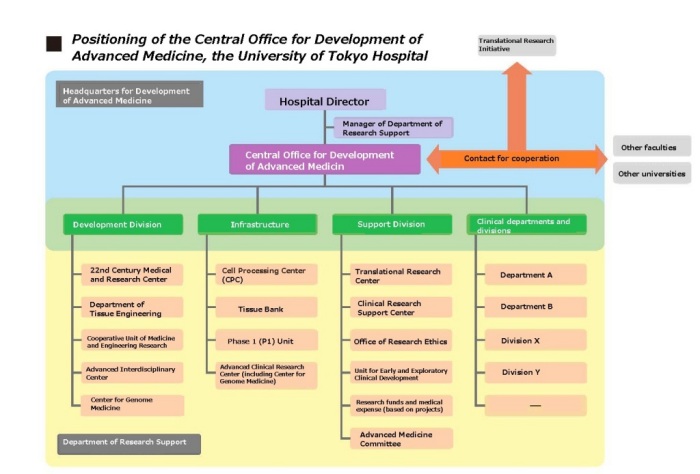
In May 2014, the Central Office for Development of Advanced Medicine officially began as a command center to devise comprehensive strategies for developing clinical research at the University of Tokyo Hospital. As an organization with a strong leadership working directly under the Hospital Director, the office is composed of the managers of the Department of Research Support, the Clinical Research Support Center, and the Translational Research Center, as well as full-time university research administrators (URAs), and others.
Research that bridges between basic research and clinical research is called “translational research (TR)”. There are various organizations to promote clinical research at the University of Tokyo Hospital. The 22nd Century Medical and Research Center, the Department of Tissue Engineering, and the Center for Genome Medicine are responsible for hands-on translational research. The Clinical Research Support Center and the Translational Research Center support translational research in terms of personnel and knowledge. The infrastructure of the Cell Processing Center (CPC) , the Phase 1 (P1) Unit, and the Advanced Clinical Research Center also plays an important role in translational research. Close cooperation with various clinical departments or divisions and the translational research divisions leads to the promotion of clinical research and establishment of advanced medicine.
To promote this cooperation, the Central Office for Development of Advanced Medicine aims to devise comprehensive strategies that focus on reinforcement of the structure of each translational research division and strengthening of cooperation among the divisions. To achieve this objective, we first investigate research activities that are carried out in this hospital and generate a database from these results. We also attempt to discover needs and seeds in clinical practice in close cooperation with the Translational Research Center and the Translational Research Initiative, which is an organization that all faculties of the University of Tokyo are involved in. Furthermore, we intend to establish a stronger organizational structure by considering the sustainability of the projects and organizations that are supported by time-limited projects of ministries and agencies, and we devise comprehensive strategies while getting an early understanding of the trends in government, ministries, and regulatory agencies, and collecting related information.
In addition, we play a foundational supporting role for research activities by researchers in this hospital in cooperation with the Administration Office. We also consult with researchers regarding the acquisition of research funds and intellectual property, as well as play a role as the administrative headquarters when they publicly apply for large-scale research projects. Furthermore, when these projects require cooperation with other departments or divisions, we serve as a contact for the cooperation.
At the same time, we also provide educational activities and practical matters on issues, such as research ethics and conflict of interest, based on the related ethics rules and guidelines.
As seen above, the duties of the Central Office for Development of Advanced Medicine are wide-ranging and always shifting on the situation. We work every day with a sense of responsibility so that the development of clinical research at the University of Tokyo Hospital will be promoted and contribute to an improvement of the quality of medical care service in Japan.
Mission of the Central Office for Development of Advanced Medicine
- Planning of comprehensive strategies for research and development
- Serving as headquarters for public applications for large-scale research projects
- Consultation about acquisition of research funds and intellectual property
- Evaluation of strategies for self-sustainability related to financial matters
- Investigation of research activities in the hospital and generation of a database
- Collection of information about clinical research from external institutions
- Control of documents related to conflict of interest