Centers & Services
Top > Centers & Services > Clinical Research Services > Clinical Research Promotion Center
Clinical Research Promotion Center
Clinical Research Promotion Center is an organization where physicians, nurses, pharmacists, clinical laboratory technologists, and other specialists promote clinical research that contributes to medical innovation and the selection of optimal medical care, while ensuring high ethical standards and scientific validity, in accordance with Good Clinical Practices (GCP), Clinical Trials Act, and the Ethical Guidelines for Medical and Health Research Involving Human Subjects, etc.
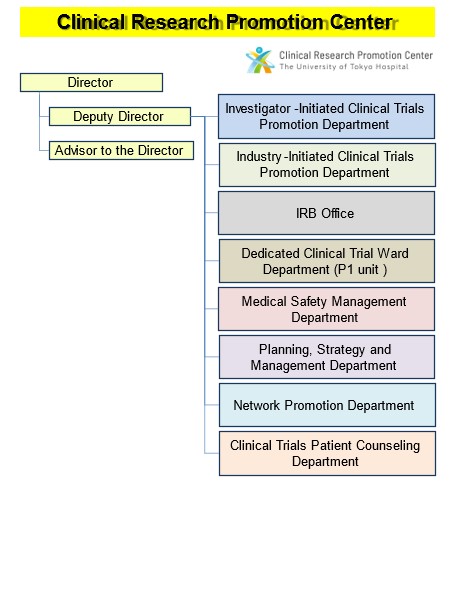
Organizational structure
The Center is composed of 8 departments.
Investigator-Initiated Clinical Trials Promotion Department
The department is responsible for: (1) secretariat duties for investigator-initiated clinical trials, advanced medical treatment, patient-initiated medical treatment and regenerative medicine; (2) Providing advice and suppor t regarding development strategy, trial design, pharmaceutical affairs, funding, intellectual property, industry-academia collaboration, etc.; (3) Providing support (study management, monitoring,safety information suppor t, data management, biostatistics, etc.) to promote investigator-initiated trials.
Industry-Initiated Clinical Trials Promotion Department
The department is responsible for: (1) Supporting the safe and smooth implementation of industry-initiated clinical trials; (2) CRC operations, study drug management, and clinical psychological examinations.
IRB Office
The office is responsible for: (1) Managing the Institutional Review Board (IRB) and coordinating with related departments; (2) Providing support for investigators who wish to be reviewed by the IRB.
Dedicated Clinical Trial Ward Department (P1 unit)
The department is responsible for: (1) The safe and smooth conduct of clinical trials related to pharmaceuticals and medical devices; (2) the recruitment of human subjects.
Medical Safety Management Department
The department is responsible for management of safety reports and collection and reporting of safety information.
Planning, Strategy and Management Department
The department is responsible for: (1) Planning for responding to authorities, acquisition of funds for projects and expansion of the Center's functions; (2) Management of the Center's budget, personnel and documents; (3) Overall management of the Center's information system, including information security; (4) Education and training for and provision of information to investigators who wish to conduct clinical research; (5) Management,education and training of clinical research trainers; (6) Management of the progress of clinical research conducted at the University of Hospital.
Network Promotion Department
The department is responsible for: (1) Secretariat duties for the National University Hospitals Clinical Research Promotion Initiative joined by 44 hospitals of 42 national universities; (2) Secretariat duties for the University of Tokyo Hospital related to the University Hospital Clinical Trial Alliance which is a regional network of national university hospitals involved in clinical research (consisting of 8 universities and 9 hospitals in the Kanto-Koshinetsu region).
Clinical Trials Patient Counseling Department
The department is responsible for providing consultation services for patients and citizens who would like to use advanced medical care and public relation activities.
Takashi Moritoyo
Departments/Divisions
Clinical Research Promotion Center
Titles
M.D. , Ph.D.
Expertise/Specialties
Clinical Pharmacology, Neurology
Research Interests
Clinical Pharmacology, Neurology
Languages
Japanese, English

